be2 bond order
We we draw the molecular orbital diagram for neutral di-beryllium we find a bond order of zero since there are four bonding electrons and four anti-bonding electrons. We we draw the molecular orbital diagram for neutral di-beryllium we find a bond order of zero since there are four bonding electrons and four anti-bonding electrons.
 |
| Using The Molecular Orbital Theory Why Does A Be2 Molecule Not Exist Quora |
The bond order then because this is a bonding molecular orbital would be.
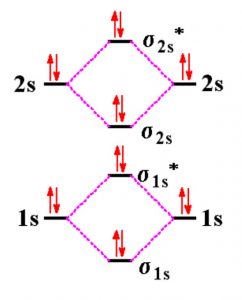
. Since each 2p x and 2p y MO contains unpaired electron therefore B 2 molecule is. How many protons and electrons in Be2 plus ION. So the bond order of B2 is equal to 1 which you can get by drawing the molecular orbital diagram and performing the equation Bond Order. Because each beryllium atoms valence shell is.
Be2 ion has 4 protons and 2 electrons What is the bond order of NO. Bond order of Be 2 is. Bond order can be calculated by the following formula rm Bond order 3 05 rm n Here n is the difference between the total number of electrons and 14 in the. A 1 B 2 C 3 D 0 Medium Solution Verified by Toppr Correct option is D Be 28σ 1s 2σ 1s 2 σ 2s 2σ 2s 2 Bond Order BO of Be 2 21440 Solve any question of.
Since bond order is zero Be2 molecule does not exist. The _valence shell configuration is 1s2 2s2. The molecular orbital electronic configurationMagnetic property. Match the items in the left column to the appropriate blanks in the sentences on the right.
Two atomic orbitals joined to form a molecular orbital with a bonding non-bonding and antibonding orbital. Reset Help 1 The bond order of Be2 is The bond order of Bez is 0 able to exist in the gas phase. 5 of bonding electrons. The bond order for Be 2 is 0 zero.
This theory deals with the generation of bonding and. Molecular Orbital Diagram for Beryllium Dimer Be2Fill from the bottom up with 4 electrons totalBonding Order is 0 meaning it does not bond and it is di. Bond order of Be 2 is. Be2s bond order is 0 zero.
See full answer below. It is diamagnetic due to the absence of1. Since B2 is paramagnetic with two electrons in order to make that bond there are indeed two half- π bonds which form what we represent improperly in line notation as a σ bond and it isnt. If these core electrons than could combine into molecular orbitals we would see a sigma one s molecular orbital.
One of the theories used to explain molecular bonding is Molecular Orbital Theory. The bond order for Be2 is 0 zero. Bond order The two boron atom is B2 molecules are linked by one covalent bond. After all what is be2s bond order.
A 1 B 2 C 3 D 0 Medium Solution Verified by Toppr Correct option is D Be 28σ 1s 2σ 1s 2 σ 2s 2σ 2s 2 Bond Order BO of Be 2 21440 Solve any question of. The valence shell of each beryllium atom is 2 s2 so there are a total of four valence shell electrons for which we. I Be _ 1s2 2s2 has 2 bonding and 2. Yes Be2-1 exists in the gas phase.
 |
| Solved Draw An Mo Energy Diagram And Predict The Bond Order Of Be2 And Be2 Do You Expect These Molecules To Exist In The Gas Phase |
 |
| Use Mo Diagrams And The Bond Orders You Obtain From Them To Quizlet |
 |
| Using The Molecular Orbital Theory Why Does A Be2 Molecule Not Exist Quora |
 |
| Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds |

Posting Komentar untuk "be2 bond order"